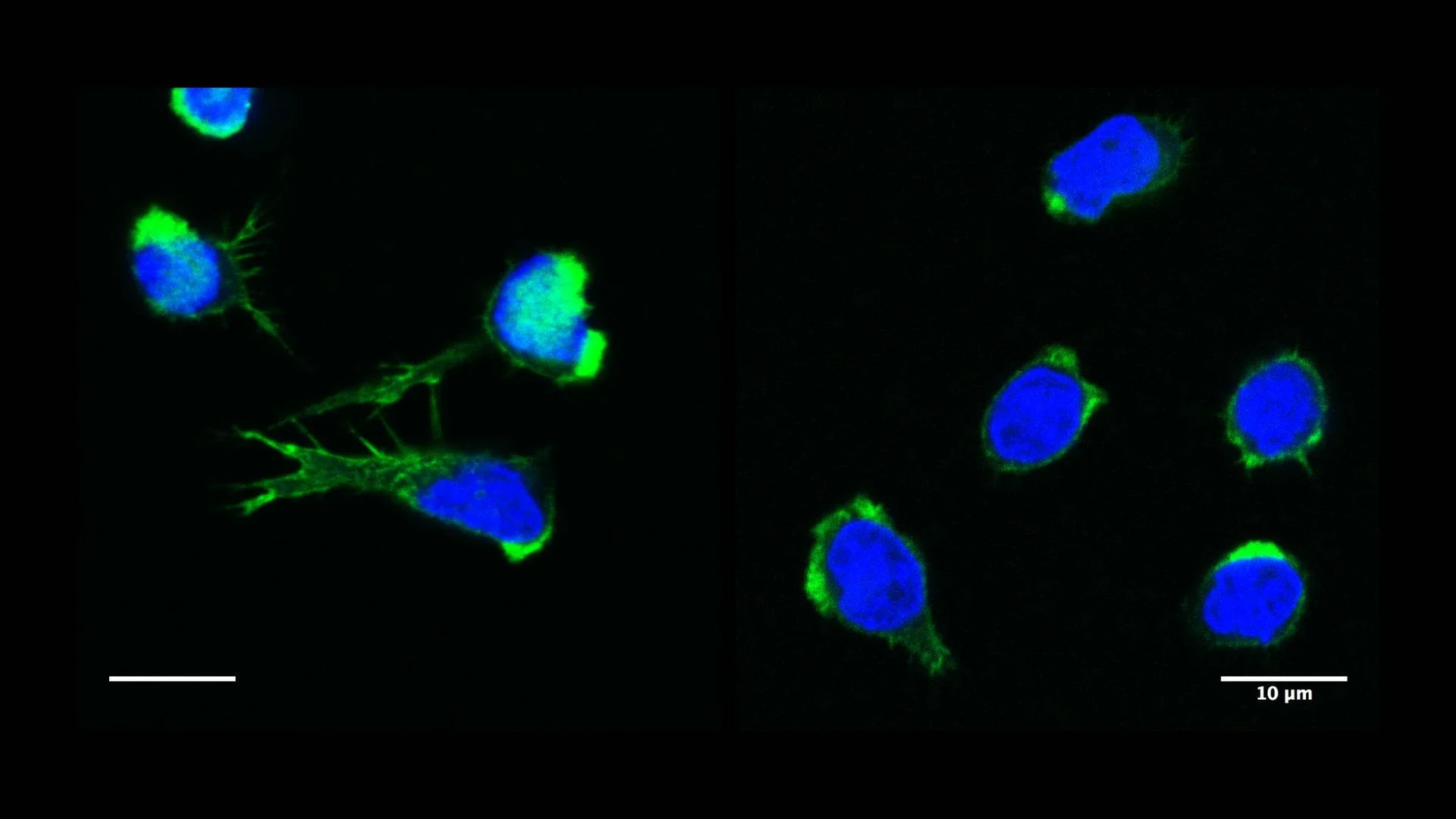
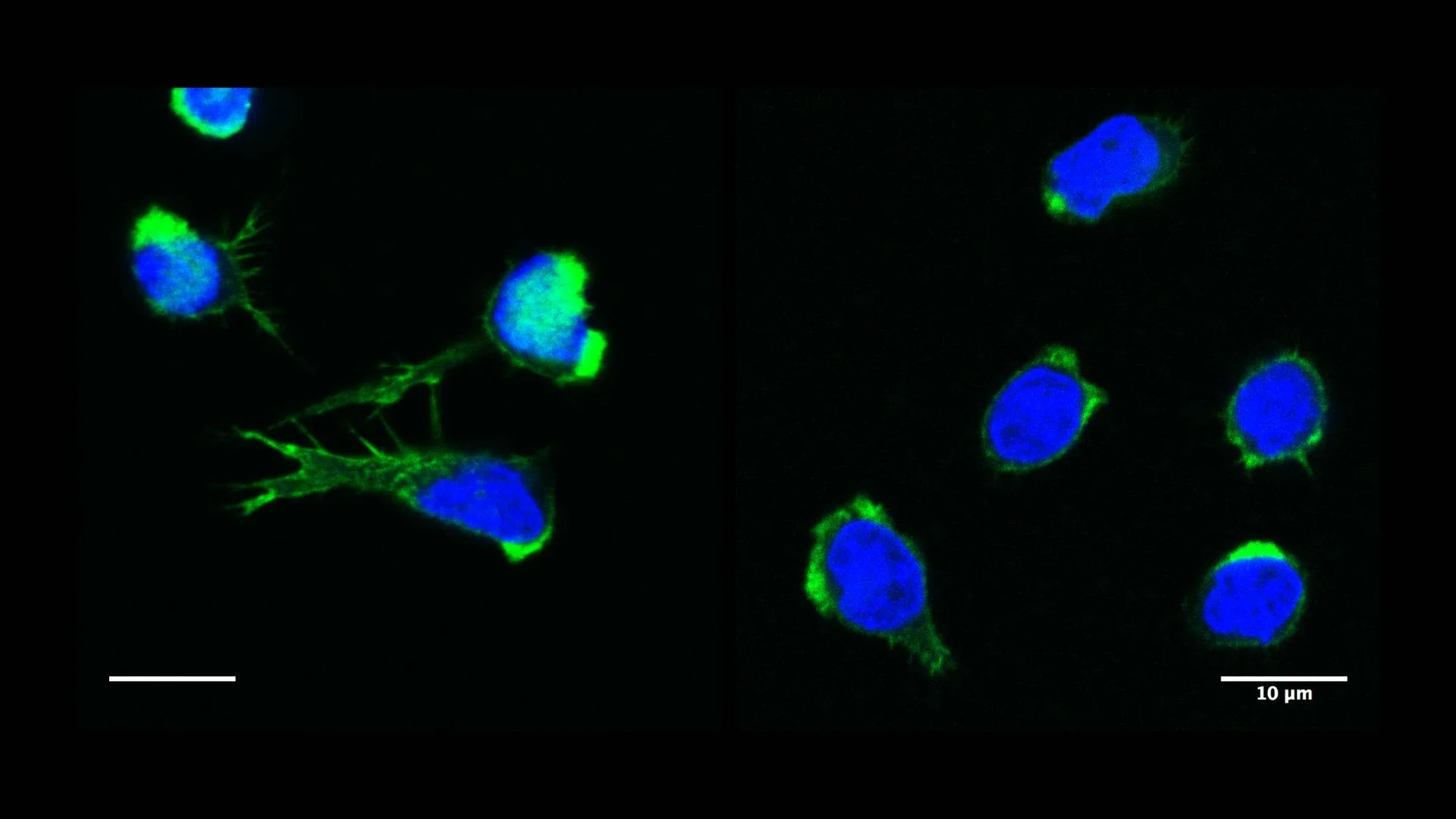
The actin cytoskeleton is composed of filaments that organize locally in higher-order networks, giving eukaryotic cells their shape and ability to move. Actin filaments link to nearly every internal organelle, guiding intracellular transport and helping maintain proper cellular balance. To allow actin networks to extend, retract, or reorganize as the cell responds to its environment, specialized actin-regulatory proteins continually assemble and disassemble these filaments. Defects in individual actin-regulatory proteins result in immune-related actinopathies, a significant cause of inborn errors of immunity (IEIs). By today, mutations in genes encoding for nearly 30 actin-regulatory proteins have been found to be associated to IEI occurrence. “By combining our respective expertise in human genetics and immune cell biology, Kaan Boztug and myself have worked over the past years at elucidating the function of some of these disease-related actin regulators.” says Loïc Dupré, Inserm Reseach Director at the Toulouse Institute for Infectious and Inflammatory Diseases, France. “This has lifted our understanding about how immune cells function.”
Inborn Errors of Immunity
Inborn immune-related actinopathies underline the importance of cytoskeleton dynamics for immune-cell function “Individuals affected show a significantly increased susceptibility to infectious diseases, but also often exhibit a variety of autoimmune and autoinflammatory symptoms“ states Prof. Kaan Boztug, head of the Clinic of Pediatric Immunology and Rheumatology at the UKB and member of ImmunoSensation2. “The underlying cellular malfunctions can be manyfold.”
Actinopathies can disrupt the normal regulation of T- and B-cells, leading to impaired immune tolerance and hence, autoimmune reactions. In myeloid cells, that are part of the innate immune system and serve as a first line of defense against infections, actinopathies can trigger excessive inflammatory responses, leading to chronic inflammation.
Implications of Immune Diversity
Not only the mechanisms underlying immune-related actinopathies are diverse. The resulting symptoms can also vary considerably despite similar actin-related mutations. “Additional genetic and environmental factors may explain why patients with similar actin-related mutations can show very different symptoms.” Prof. Boztug explains.
In future research projects, mouse models may help distinguish defects caused directly by actin mutations from those influenced by other factors. „To fully understand immune dysregulation in these disorders, we need to shift the view from individual cells or genes and adopt a systems-level approach that considers how multiple cell types interact within the immune system.” Prof. Boztug adds. Future studies will need to include larger groups of patients to better understand the mechanisms behind immune dysregulation and to identify biomarkers that can predict who is at risk. At the same time, developing more targeted therapies will be essential to turn the growing cellular and molecular understanding of these disorders into improved clinical care for affected patients.