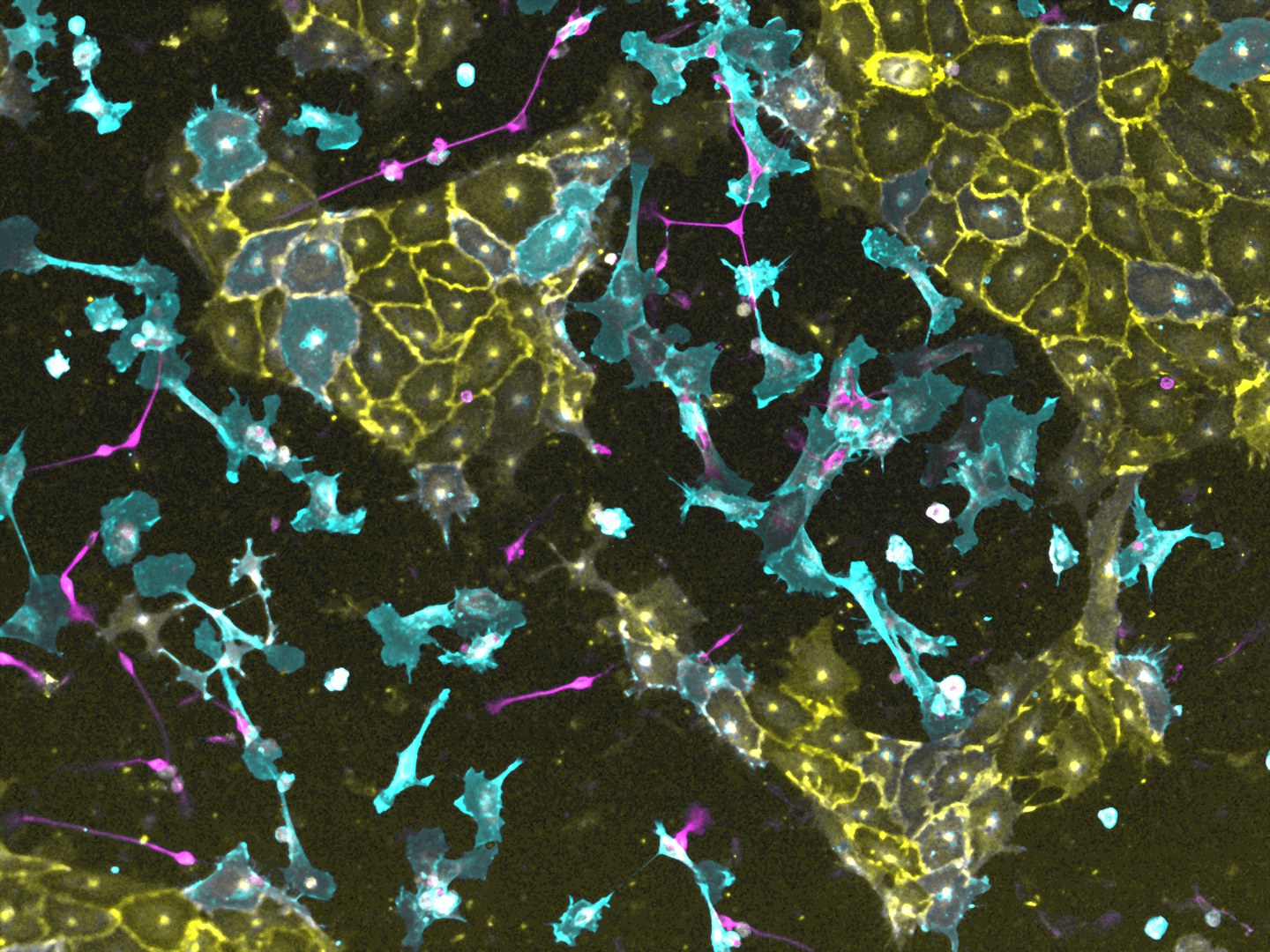
The researchers used human induced pluripotent stem cells (iPS), which were reprogrammed from connective tissue cells into a quasi-embryonic state. In principle, iPS cells can be used to obtain all kinds of differentiated cells, from neurons to blood vessel cells, with each recipe being individually adapted. "Most differentiation protocols are very laborious and complicated. It's not possible to obtain different cell types from iPS simultaneously and in a controlled manner in a single culture," explains Prof. Dr. Volker Busskamp, who works at the Eye Clinic and the ImmunoSensation2 Cluster of Excellence at the University of Bonn the Excellence Cluster Physics of Life (PoL) and at the CRTD at TU Dresden.
Together with a team from Harvard University, TU Dresden and the University of Bonn, he aimed to replace the complicated procedures with simple "recipes". Using a large-scale screening process, the researchers found a total of 290 DNA-binding proteins that quickly and efficiently reprogram stem cells into target cells. The researchers were able to demonstrate that just a single transcription factor is sufficient in each case to derive differentiated neurons, connective tissue, blood vessel and glial cells from the stem cells within four days. The latter coat neurons as "insulators".
A genetic switchboard for stem cell differentiation
Using automated procedures, the researchers introduced the DNA sequence for the respective transcription factor and other control elements into the stem cell genome. The transcription factors could be activated by adding a small molecule, causing some of the transgenic stem cells to be converted into differentiated cells. It was then possible to distinguish and automatically sort stem cells and differentiated cells using cell markers. The researchers subsequently investigated how much of a certain transcription factor was present in the differentiated cells compared to the stem cells. "The greater the difference, the more important the respective transcription factor seems to be for the conversion of iPS into differentiated cells," explains Busskamp.
The team used this method to test a total of 1732 potential transcription factors on three different stem cell lines. The researchers found an effect for 290 different transcription factors that caused the iPS to convert into differentiated cells. This is new territory, because this property of the iPS programming of 241 of the discovered transcription factors was previously unknown. Using the example of neurons, connective tissue, blood vessel and glial cells, the researchers performed various tests to show that the converted cells are very similar to human body cells in their functional ability.
The results open new possibilities in research
"The advantage of the identified transcription factors is that they are able to convert iPS into body cells particularly quickly and easily and that they can potentially also be used to form more complex tissues," says Busskamp. What took weeks or even months now happens within days. Instead of costly and time-consuming protocols, a single transcription factor is sufficient for the hits identified in mass screening.
"These results open new possibilities," says Prof. Dr. George M. Church of Harvard University. "The variety, simplicity and speed of stem cell programming using transcription factors makes stem cell research possible on a large scale. Worldwide, 50 other groups are already working with our programmable stem cell lines and with the transcription factor library”. The two lead authors Alex H.M. Ng and Parastoo Khoshaklagh from Harvard University have now founded the start-up GC Therapeutics in Cambridge (USA), which provides programmable stem cells with customized, integrated transcription factors.
"The cooperation between the different research institutions was very successful, because the different disciplines complemented and interlinked with each other very well," says Busskamp. Researchers worldwide can now use the transcription factor resource that is available by the non-profit organization Addgene.
Particularly as an expert in degenerative retinal diseases, Busskamp sees great potential for stem cell technology in ophthalmology. "For diseases in which the retina degenerates, such as age-related macular degeneration (AMD), there is hope that at some point, it will be possible to replace the affected photoreceptors with the help of iPS conversion," says Busskamp. "My team is working towards this goal."
Funding:
Prof. Busskamp is a Freigeist Fellow of the Volkswagen Foundation, receives funding from the German Research Foundation and is supported by an ERC-Starting Grant of the European Union.
Publication: Alex H.M. Ng, Parastoo Khoshakhlagh, Jesus Eduardo Rojo Arias, Giovanni Pasquini, Kai Wang, Anka Swiersy, Seth L. Shipman, Evan Appleton, Kiavash Kiaee, Richie E. Kohman, Andyna Vernet, Matthew Dysart, Kathleen Leeper, Wren Saylor, Jeremy Huang, Amanda Graveline, Jussi Taipale, David E. Hill, Marc Vidal, Juan M. Melero-Martin, Volker Busskamp, George M. Church: A comprehensive library of human transcription factors for cell fate engineering, Nature Biotech, DOI: 10.1038/s41587-020-0742-6
Media contact:
Prof. Dr. Volker Busskamp
Universitäts-Augenklinik Bonn
Tel. +49-(0)228- 28713687
E-mail: volker.busskamp@uni-bonn.de
Zentrum für Regenerative Therapien Dresden (CRTD) der TU Dresden
Tel. +49-(0)351-45882015
E-mail: volker.busskamp@tu-dresden.de