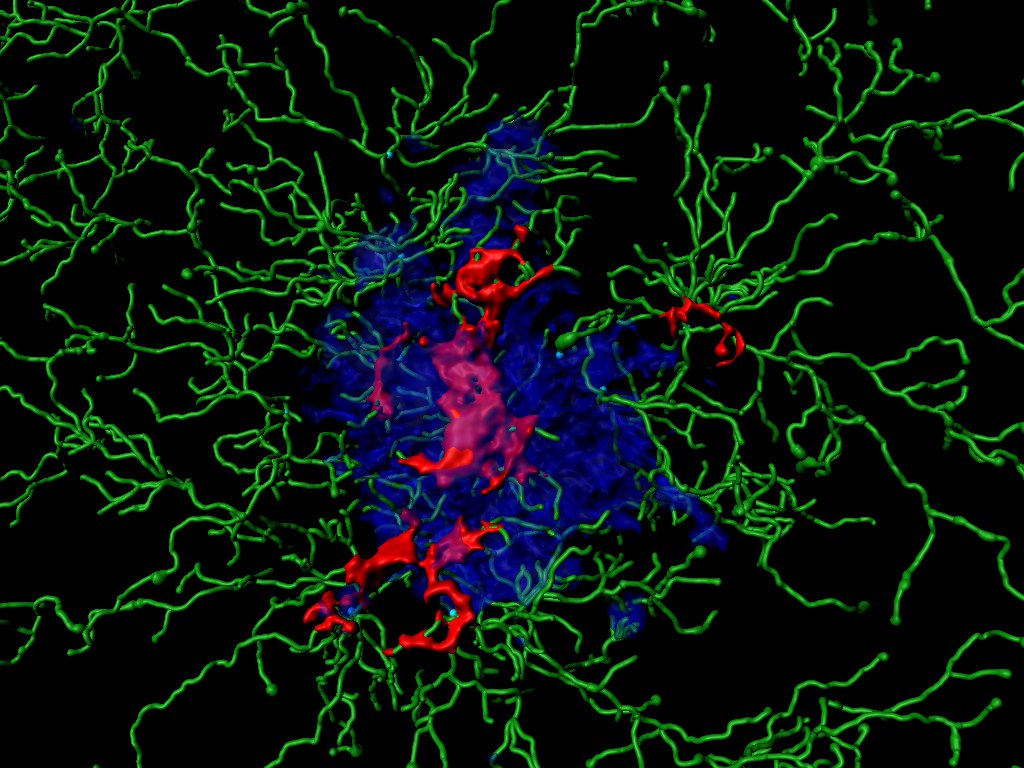
Alzheimer’s disease is a devastating neurodegenerative condition ultimately leading to dementia. An effective treatment does not yet exist. The disease is associated with the aberrant aggregation of small proteins called “Amyloid-beta” (Aß) that accumulate in the brain and appear to harm neurons. In recent years, studies revealed that deposits of Aß, known as “plaques”, trigger inflammatory mechanisms by the brain’s innate immune system. However, the precise processes that lead to neurodegeneration and progression of pathology have thus far not been fully understood.
“Deposition and spreading of Aß pathology likely precede the appearance of clinical symptoms such as memory problems by decades. Therefore, a better understanding of these processes might be a key for novel therapeutic approaches. Such treatments would target Alzheimer’s at an early stage, before cognitive deficits manifest,” says Prof. Michael Heneka, a senior researcher at the DZNE and Director of the Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn.
An Inflammatory Cascade
Prof. Heneka, who is also involved in the cluster of excellence “ImmunoSensation” at the University of Bonn, and coworkers have been investigating the role of the brain’s immune response in the progression of Aß pathology for some time already. Previous work by the group that was published in Nature in 2013, had established that the molecular complex NLRP3, which is an innate immune sensor, is activated in brains of Alzheimer’s patients and contributes to the pathogenesis of Alzheimer’s in the murine model. NLRP3 is a so-called inflammasome that triggers production of highly pro-inflammatory cytokines. Furthermore, upon activation, NLRP3 forms large signaling complexes with the adapter protein ASC, which are called “ASC specks” that can be released from cells. “The release of ASC specks from activated cells has so far only been documented in macrophages and their relevance in disease processes has so far remained a mystery,” says Prof. Eicke Latz, director of the Institute of Innate Immunity and member of the cluster of excellence “ImmunoSensation” at the University of Bonn
Connection between Inflammation and Neurodegeneration
In the current study, it was demonstrated that ASC specks are also released from activated immune cells in the brain, the “microglia”. Moreover, the findings provide a direct molecular link to classical hallmarks of neurodegeneration. “We found that ASC specks bind to Aß in the extracellular space and promote aggregation of Aß, thus directly linking innate immune activation with the progression of pathology,“ Heneka says.
Novel Approach for Therapy?
This view is supported by a series of experiments in mouse models of Alzheimer’s disease. In these, the researchers investigated the effects of ASC specks and its component, the ACS protein, on the spreading of Aß deposits in the brain.
“Additionally, analysis of human brain material indicates at several levels that inflammation and Aß pathology may interact in a similar fashion in humans. Together our findings suggest that brain inflammation is not just a bystander phenomenon, but a strong contributor to disease progression,” Heneka says. “Therefore, targeting this immune response will be a novel treatment modality for Alzheimer’s.”
Publication: “Microglia-derived ASC specks cross-seed ß-amyloid in Alzheimer’s disease”, Carmen Venegas, Sathish Kumar et al., Nature (2017), DOI: 10.1038/nature25158
Media Relations
Dr. Marcus Neitzert
DZNE, Media Relations
Phone: +49 228 43302-267
Email: marcus.neitzert@dzne.de
Johannes Seiler
University of Bonn, Press and PR
Phone: +49 228 73-4728
Email: j.seiler@uni-bonn.de